May 12, 2025 by Caitlin Hayes, Cornell University
Collected at: https://techxplore.com/news/2025-05-sunlight-powered-mimics-power-carbon.html
Current methods of capturing and releasing carbon are expensive and so energy-intensive they often require, counterproductively, the use of fossil fuels. Taking inspiration from plants, Cornell researchers have assembled a chemical process that can power carbon capture with an energy source that’s abundant, clean and free: sunlight.
The research could vastly improve current methods of carbon capture—an essential strategy in the fight against global warming—by lowering costs and net emissions.
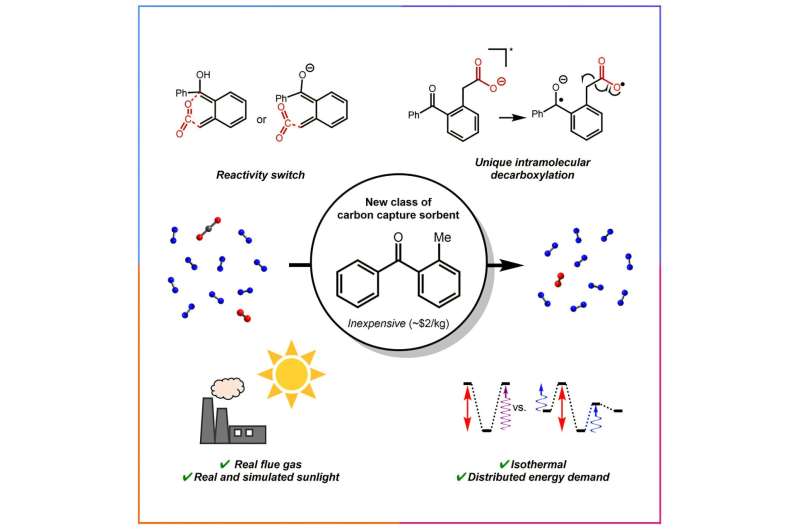
In the study, published in Chem, researchers found that they can separate carbon dioxide from industrial sources by mimicking the mechanisms plants use to store carbon, using sunlight to make a stable enol molecule reactive enough to “grab” the carbon.
They also used sunlight to drive an additional reaction that can then release the carbon dioxide for storage or reuse. It’s the first light-powered separation system for both carbon capture and release. Graduate student Bayu Ahmad, M.S. ’23, is first author.
“From a chemistry standpoint, this is totally different than what anybody else is doing in carbon capture,” said senior author Phillip Milner, associate professor of chemistry and chemical biology in the College of Arts and Sciences. “The whole mechanism was Bayu’s idea, and when he originally showed it to me, I thought it would never work. It totally works.”
Carbon dioxide is challenging to capture because it’s inert, Milner said, which has led researchers and industry to amines—organic, ammonia-derived compounds that contain nitrogen—which react selectively with carbon dioxide and can pull it from mixtures of many compounds. But amines are not stable in the presence of oxygen and don’t last, requiring the energy-intensive production of more and more amines.
“From the beginning, our lab has tried to think about how we can use our intuition as chemists to find alternative pathways to carbon dioxide capture,” Milner said. “We basically have a motto of ‘anything but amines.'”
The carbon capture reaction uses the same mechanism the enzyme RuBisCo, crucial for photosynthesis, uses to fix carbon in plants. To release the carbon, the researchers changed the pH to enable decarboxylation, or the removal of a carboxyl group. They used an inexpensive sorbent, 2-methylbenzophenone, and found the rate of carbon capture in the new system was equal to or better than other light-driven technologies. The system also did not require additional cooling between the release and capture steps, a major limitation of other carbon capture and release methods.
The researchers tested the system using flue samples from Cornell’s Combined Heat and Power Building, an on-campus power plant that burns natural gas, and found it was successful in isolating carbon dioxide. Milner said this step was significant, as many promising methods for carbon capture in the lab fail when up against real-world samples with trace contaminants.
Milner and his team envision staging the reaction on what looks like a solar panel—but one that would capture carbon instead of generate electricity. As an inaugural Semlitz Family Sustainability Fellow in the Cornell Atkinson Center for Sustainability, Ahmad is working with partners in the Cornell SC Johnson College of Business to explore commercialization.
“We’d really like to get to the point where we can remove carbon dioxide from air, because I think that’s the most practical,” Milner said. “You can imagine going into the desert, you put up these panels that are sucking carbon dioxide out of the air and turning it into pure high-pressure carbon dioxide. We could then put it in a pipeline or convert it into something on-site.”
Milner’s lab is also exploring how the light-powered system could be applied to other gases, as separation drives 15% of global energy use.
“There’s a lot of opportunity to reduce energy consumption by using light to drive these separations instead of electricity,” Milner said.
Milner is part of an effort to make flue samples from Cornell’s Combined Heat and Power Building available to researchers and startups studying carbon capture at Cornell and beyond.
“Getting real flue gas from industry is really difficult, because companies don’t want people to know what’s coming out of their power plants,” Milner said. “But Cornell is not a company—so this is something unique that we can offer and that we hope will be operational in the next year.”
More information: Bayu I.Z. Ahmad et al, A fully light-driven approach to separate carbon dioxide from emission streams, Chem (2025). DOI: 10.1016/j.chempr.2025.102583
Journal information: Chem
There are certainly plenty of details like that to take into consideration. That could be a nice level to deliver up. I provide the thoughts above as basic inspiration but clearly there are questions just like the one you bring up where the most important thing might be working in trustworthy good faith. I don?t know if best practices have emerged round issues like that, but I am sure that your job is clearly recognized as a good game. Each girls and boys really feel the affect of only a second’s pleasure, for the remainder of their lives.

Leave a Reply