January 16, 2026 by Ingrid Fadelli, Phys.org
Collected at: https://phys.org/news/2026-01-imaging-technique-captures-ultrafast-electron.html
During chemical reactions, atoms in the reacting substances break their bonds and re-arrange, forming different chemical products. This process entails the movement of both electrons (i.e., negatively charged particles) and nuclei (i.e., the positively charged central parts of atoms). Valence electrons are shared and re-arranged between different atoms, creating new bonds.
The movements of electrons and nuclei during chemical reactions are incredibly fast, in many cases only lasting millionths of a billionth of a second (i.e., femtoseconds). Yet reliably tracking and understanding these movements could help to shed new light on how specific molecules are formed, as well as on the underpinnings of quantum mechanical phenomena.
Researchers at Shanghai Jiao Tong University recently introduced a new approach to observe chemical reactions as they unfold, precisely tracking the movement of electrons and atomic nuclei as a molecule breaks apart. This strategy, outlined in a paper published in Physical Review Letters, was successfully used to image the photodissociation of ammonia (NH₃), the process in which a NH₃ molecule absorbs light and breaks down into smaller pieces.
“The investigation of electron dynamics holds substantial significance for advancing fundamental physics and for applied research in materials and chemical sciences,” Dao Xiang, senior author of the paper, told Phys.org. “With the advent of Nobel Prize–winning attosecond technology, probing ultrafast electron dynamics has entered a new era.”

Real-time imaging of valence electron dynamics in NH3 photodissociation. Credit: Jiang at Shanghai Jiao Tong University.
Tracking the movement of electrons in space has been a long-standing objective in various scientific fields. While ultrafast diffraction techniques are widely used for studying molecular dynamics in real space, they have so far proved to be ineffective for the precise tracking of electron movements.
“Through recent advancements in electron diffraction methods, we have successfully achieved simultaneous improvements in both temporal and spatial resolution to an exceptional level,” said Xiang.
“It is therefore a significant step to pursue the real-space imaging of electron dynamics. This endeavor will not only drive fundamental methodological progress in electron diffraction for probing electronic motion, but it will also deepen our understanding of electron dynamics.”
Tracking valence electron and hydrogen dynamics in ammonia
Xiang and his colleagues introduced a new promising methodology to track the valence electron and hydrogen dynamics, which builds on recently demonstrated improvements to diffraction techniques. To test the potential of their approach, they used it to observe electron and hydrogen movements in NH3 molecules during their photodissociation.
“We used a ~200 nm laser pulse to pump the NH3 molecules to the excited electronic state,” explained Xiang. “The coupled electronic and nuclear dynamics in photoexcitation of NH3 molecules is probed by ultrafast electron diffraction (UED), where a delayed MeV electron pulse is scattered by the Coulomb potential when interacting with the target molecules, and the diffraction pattern recorded by the detector encodes the charge density and structural information of target molecules.
“We performed the charge pair distribution function (CPDF) analysis to the experimental diffraction intensity, which allows concurrent imaging of both valence electrons and hydrogen dynamics.”
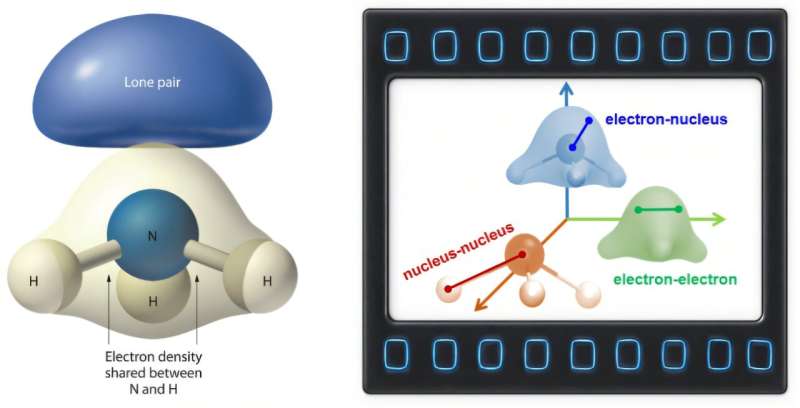
The researchers combined this analysis with calculations of the diffraction intensity. This allowed them to separately visualize the dynamical evolution of electron-electron, nucleus-nucleus and electron-nucleus charge pairs during NH3 photodissociation. Using UED, the team was also able to capture the density of electrons and the structural dynamics of nuclei in real-time.
“The high spatio-temporal resolution and high signal-to-noise ratio of our UED experimental setup enabled the imaging of hydrogen motion, which is well-known to be challenging due to its low scattering cross section and fast motion,” said Xiang. “Triggered by the pump pulse, an electron in the lone pair orbital of N atom is excited to the N-H anti-bonding sigma orbital, leading to the NH3 umbrella motion and N-H dissociation.”
Xiang and his colleagues’ experimental approach allowed them to concurrently track the movements of valence electrons and nuclei in real space. A novelty of their work is the use of CPDF analysis, which allowed them to simultaneously observe electron and nucleus dynamics, overcoming the limitations of previous analysis tools that focused solely on the movements of atoms.
“The large proportion of valence electrons in NH3 makes the electronic density largely different from that predicted by the independent atom model,” said Xiang. “Our analysis successfully revealed the abundant electronic dynamics encoded in the UED signal, including electronic orbital transition, electronic density evolution dynamics and electron-electron correlation.”
A new tool for chemistry and quantum science
This recent study demonstrates the potential of UED for studying the underpinnings of specific chemical reactions. Apart from NH3 photodissociation, their experimental setup is also used to study other molecules, potentially yielding new insight into various chemical and physical processes.
“Our work pushes the limit of UED to unravel the relatively subtle signal, which are often overshadowed by the more pronounced scattering signals contributed by the structural change of heavy atoms,” said Xiang.
In the future, the UED-based approach introduced by this research group could also be combined with other high-precision techniques, such as ultrafast X-ray diffraction imaging and attosecond spectroscopy. Collectively, these techniques could be used to capture various rapid and invisible processes in even greater detail.
“We will now extend our methodology to other molecular systems, demonstrating the capability of electron diffraction to detect signatures of valence electron rearrangement even in complex organic molecules,” added Xiang.
“Subsequently, we will advance techniques for extracting spatial electronic information to obtain the most comprehensive real-space picture of electrons. Ultimately, through the continuous improvement and upgrading of our instrumentation, we will progressively push electron diffraction studies into the attosecond temporal domain.”
Publication details
Tianyu Wang et al, Probing Valence Electron and Hydrogen Dynamics Using Charge-Pair Imaging with Ultrafast Electron Diffraction, Physical Review Letters (2025). DOI: 10.1103/kny2-gf5x. On arXiv: DOI: 10.48550/arxiv.2506.21047
Journal information: Physical Review Letters , arXiv

Leave a Reply