April 23, 2025 by Bob Yirka , Phys.org
Collected at: https://phys.org/news/2025-04-explanation-molecular-hydrogen-space.html
Two materials physicists at The University of Sydney have found a possible explanation for the huge amount of molecular hydrogen in space. In their study published in the journal Communications Chemistry, Yuzhen Guo and David McKenzie tested the possibility of space dust serving as a catalyst to allow hydrogen atoms to merge into hydrogen molecules in space.
Astronomers have puzzled over the abundance of molecular hydrogen in space for many years. This is because it has been difficult to envision a scenario where two hydrogen atoms floating in the vastness of space would smack into one another and bond. In this new study, the researchers wondered if space dust might provide the solution.
To test the possibility, the researchers used fullerenes as a stand-in for space dust. They chose the 60-carbon-atom spheroidal molecules because they share so many characteristics of space dust. Also, prior research has suggested that there are many C60 molecules floating around in space.
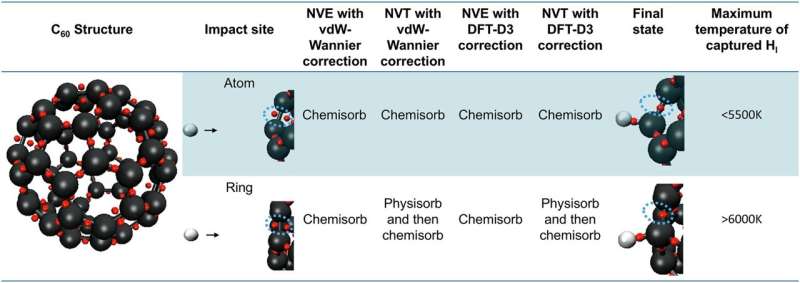
To test the idea of space dust serving as a catalyst for the creation of hydrogen molecules, the researchers created a model on a computer showing the structure and characteristics of a fullerene shaped somewhat like a football. Then they simulated what might happen if two hydrogen atoms were to run into the fullerene under two different scenarios.
Under the first scenario, two hydrogen atoms that were already barely stuck to the fullerene moved around on its surface until they collided with one another. In the second scenario, a hydrogen atom floating in space collided with another hydrogen atom that was attached to the fullerene.
The researchers found that the two hydrogen atoms would bond, allowing the formation of a hydrogen molecule under both scenarios. They also noted that no reverse reaction occurred due to the energy being released from the collision because it was absorbed by the fullerene rather than the newly formed hydrogen molecule.
They also found that such bonding could occur in medium temperatures of 50 K and at temperatures down to 10 K. They conclude that the formation of hydrogen molecules in space can also occur under higher energies and temperatures.
More information: Yuzhen Guo et al, Ab-initio dynamic study of mechanisms for dust-mediated molecular hydrogen formation in space, Communications Chemistry (2025). DOI: 10.1038/s42004-025-01489-z
Journal information: Communications Chemistry
Good ?V I should definitely pronounce, impressed with your site. I had no trouble navigating through all tabs as well as related info ended up being truly easy to do to access. I recently found what I hoped for before you know it at all. Reasonably unusual. Is likely to appreciate it for those who add forums or something, site theme . a tones way for your client to communicate. Nice task..

Leave a Reply