
January 7, 2026 by Chalmers University of Technology
Collected at: https://techxplore.com/news/2026-01-solar-hydrogen-efficiently-platinum-required.html
A research team led by Chalmers University of Technology, Sweden, has presented a new way to produce hydrogen gas without the scarce and expensive metal platinum. Using sunlight, water and tiny particles of electrically conductive plastic, the researchers show how the hydrogen can be produced efficiently, sustainably and at low cost.
Hydrogen plays a key role in the global pursuit of renewable energy. Although its use produces only water as a by-product, significant challenges remain before hydrogen can be produced both on a large scale and in an environmentally friendly way.
A major challenge is the use of the metal platinum as a co-catalyst when sunlight and water are used to produce hydrogen. Earth’s reserves of platinum are limited, and extraction is associated with risks to both the environment and to human health. Moreover, the production is concentrated in only a few countries, for example, South Africa and Russia.
In a new study, published in Advanced Materials, a research team led by Professor Ergang Wang at Chalmers, shows how solar energy can be used to produce hydrogen gas efficiently—and completely without platinum.
The process, Chalmers researcher Alexandre Holmes explains, involves quantities of tiny particles of electrically conductive plastic. Immersed in water, the particles interact both with sunlight and with their surroundings.
“Developing efficient photocatalysts without platinum has been a long-standing dream in this field. By applying advanced materials design to our conducting-plastic particles, we can produce hydrogen efficiently and sustainably without platinum—at radically lower cost, and with performance that can even surpass platinum-based systems,” says Holmes, who together with Jingwen Pan from Jiefang Zhu’s group at Uppsala University, is the joint first author of the paper.
Cured fear of water behind the success
Efforts to overcome the platinum bottleneck have been underway for several years in Wang’s research group at Chalmers.
The key to the new approach lies in advanced materials design of the electrically conductive plastic used in the process. This type of plastic, known as conjugated polymers, absorbs light efficiently, but is typically less compatible with water.
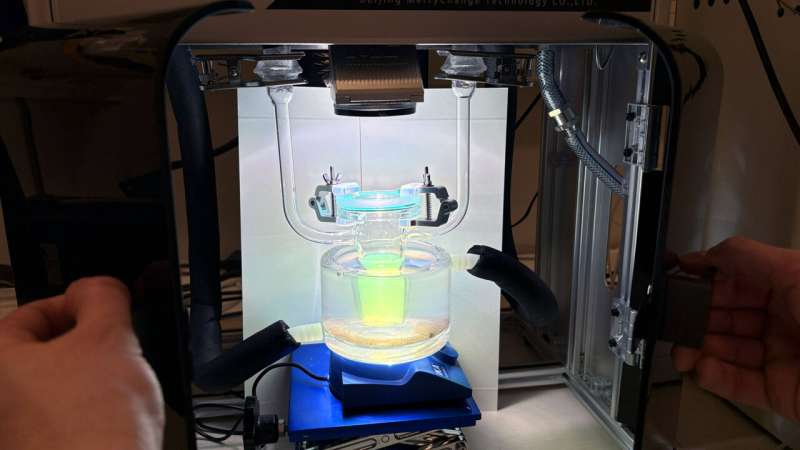
New research advances open the door to efficient hydrogen production from solar energy—without using the scarce metal platinum. In the reactor at a chemistry laboratory at Chalmers University of Technology, Sweden, it is easy to see with the naked eye how hydrogen gas is formed through photocatalysis. When a lamp, simulating sunlight, is directed at a beaker of water containing nanoparticles of electrically conductive plastic, small hydrogen gas bubbles almost immediately begin to rise through the water. The gas is then guided through tubes to a hydrogen storage container, where the amount of hydrogen produced can be read in real time. Credit: Chalmers University of Technology | Mia Halleröd Palmgren
By adjusting the material properties at the molecular level, the researchers made the material much more water compatible.
“We also developed a way to form the plastic into nanoparticles that can enhance the interactions with water and boost the light-to-hydrogen process. The improvement comes from more loosely packed, more hydrophilic polymer chains inside the particles,” says Holmes.
In the reactor at the chemistry laboratory at Chalmers, bubbles of hydrogen gas can be easily seen with the naked eye as they form—showing that photocatalysis is happening efficiently.
When a lamp with simulated sunlight is directed at a beaker of water containing the nanoparticles, small bubbles of hydrogen gas almost immediately begin to form and rise through the water. The bubbles are collected and guided through tubes to a storage container, and the amount of gas produced can be monitored in real time.

Specially designed nanoparticles of electrically conductive plastic (here in water) are a key ingredient in the researchers’ new recipe for how hydrogen from solar energy can be produced efficiently without the expensive scarce metal platinum. The nanoparticles interact with the light around them, causing them to absorb different colors and take on all the shades of the rainbow. Through advanced material design, a research team, led by Chalmers, has changed the properties of the plastic and its nanoparticles in water, which greatly improves the chemical process when hydrogen is produced from sunlight. Credit: Chalmers University of Technology | Mia Halleröd Palmgren
“With as little as one gram of the polymer material, we can produce 30 liters of hydrogen in one hour,” says Holmes.
Not only that, a recently published breakthrough from research colleagues at Chalmers shows that the electrically conductive plastic can also be produced without the use of harmful chemicals and in a much more cost-effective way.
Avoiding another expensive ingredient: Vitamin C
The next major step for Wang’s group will be to make the hydrogen process work using only sunlight and water, without any added helper chemicals.
Currently, they use vitamin C, which acts as a so-called sacrificial antioxidant. By donating electrons, it prevents the reaction from stalling, which in the laboratory can show high hydrogen production rates.
To realize truly sustainable solar hydrogen, Professor Wang explains, the goal is to split water molecules into hydrogen and oxygen simultaneously, with sunlight and water as the only inputs.
“Removing the need for platinum in this system is an important step towards sustainable hydrogen production for society. Now we are starting to explore materials and strategies aimed at achieving overall water splitting without additives. That will need a few more years, but we believe we are on the right track,” says research leader Ergang Wang, professor at the Department of Chemistry and Chemical Engineering at Chalmers.
More information: Alexandre Holmes et al, Highly Efficient Platinum‐Free Photocatalytic Hydrogen Evolution From Low‐cost Conjugated Polymer Nanoparticles, Advanced Materials (2025). DOI: 10.1002/adma.202507702
Journal information: Advanced Materials

Leave a Reply